We conduct a wide range of projects on organometallics, ligand design, main group elements chemistry and asymmetric synthesis. All of these projects aim to develop and understand new useful reactions, which could solve challenging problems in synthesis and catalysis.
Catalysis is a global and essential area of science. Principles of catalysis lay in the heart of biological action of enzymes in our body, utilization of sustainable energy, development of environmentally benign processes, preparation of polymers, fertilizers, medicines among many others. It allows unique and selective pathways for conversion of simple compounds to complex materials with minimal waste production and energy input. It is commonly accepted, that the catalyst reduces energy required for the process, so it enables chemical and biological processes to run at ambient conditions. However, one of the most exiting assets is that the catalysts enable completely novel, mechanistically different, transformations which are otherwise impossible. This is exactly the theme of activity in our research group.
We always begin our studies from very fundamentals. We design and study unique metal-based and metal-free systems, as a basis for discovery of unusual type of chemical bonding, novel chemical properties and reactivity. We truly believe that only a deep understanding of intimate chemical reactivity and mechanistic details of the reaction, fundamental studies of physical and chemical properties of the systems can bring a qualitative breakthrough and pave new directions in catalysis.
The following representative projects are currently being investigated in our group:
- Nitrenium Salt: Cationic Ligand and Nitrogen Based Lewis Acid
-
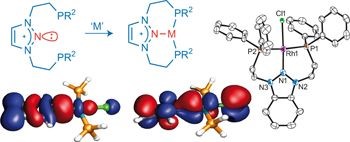 Nitrenium ligand: nitrogen based analog of carbene. Discovery of persistent and N-heterocylcic carbenes has been among the most influencing developments in coordination chemistry during the last few decades. As ligands to transition metals, NHCs have found wide appliactions in organometallic chemistry and catalysis. The outburst of NHC-based chemistry evoked much interest in preparation of main-group NHC-analogs (in which the central carbon atom is replaced by another element), and various counterparts of 13-15 groups (e.g., sillylene, phosphenium, etc.) have been proved as ligands to transition metals. The intriguing missing link in this series of ligands was the nitrogen counterpart, the N-Heterocyclic Nitrenium ion. Our group designed and developed a family of nitrenium ligands, and succeeded to demonstrate, for the first time, that nitrenium cations, isoelectronic and isostructural to ubiquitous N-heterocyclic carbenes, can serve as ligands for transition metals. For further details of our experimental and computational studies on these unique cationic ligands, read: Nature Chem. 2011, 5, 525, Chem. Sci. 2014, 5, 1305, Chem. Eur. J. 2015, 21, 7099, Organometallics, 2019, 38, 2494, and highlights in Chem. & Eng. News 2011, 89, 26, Chemistry World (January 10, 2014).
Nitrenium ligand: nitrogen based analog of carbene. Discovery of persistent and N-heterocylcic carbenes has been among the most influencing developments in coordination chemistry during the last few decades. As ligands to transition metals, NHCs have found wide appliactions in organometallic chemistry and catalysis. The outburst of NHC-based chemistry evoked much interest in preparation of main-group NHC-analogs (in which the central carbon atom is replaced by another element), and various counterparts of 13-15 groups (e.g., sillylene, phosphenium, etc.) have been proved as ligands to transition metals. The intriguing missing link in this series of ligands was the nitrogen counterpart, the N-Heterocyclic Nitrenium ion. Our group designed and developed a family of nitrenium ligands, and succeeded to demonstrate, for the first time, that nitrenium cations, isoelectronic and isostructural to ubiquitous N-heterocyclic carbenes, can serve as ligands for transition metals. For further details of our experimental and computational studies on these unique cationic ligands, read: Nature Chem. 2011, 5, 525, Chem. Sci. 2014, 5, 1305, Chem. Eur. J. 2015, 21, 7099, Organometallics, 2019, 38, 2494, and highlights in Chem. & Eng. News 2011, 89, 26, Chemistry World (January 10, 2014). -
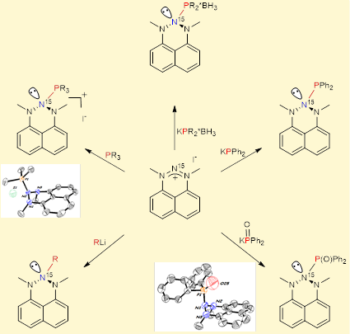 First example of Nitrogen-based Lewis acid. Lewis acids and their interactions are among the most fundamental facets of chemistry. Many chemical elements can serve as the central atom of Lewis acids (e.g., boron, aluminum, phosphorous, etc.). Nitrogen is associated with Lewis bases due to a lone pair of electrons. We discovered a first X-ray supported example of Lewis acids centered on nitrogen atom. Our novel class of Lewis acids is robust, stable and stereoelectronically modifiable, which make them as a very attractive platform for their employment in Lewis acid catalysis, frustrated Lewis pairs chemistry, small molecules activation, etc. Based on the reactivity of these new Lewis acids, we prepared, for the first time, cyclic triazanes – a class of organic compounds sequentially bearing three all-saturated nitrogen atoms (N-N-N motif). We envision very interesting and unusual chemistry (which is under intensive investigation in our labs) of this novel class of nitrogen materials. For more details, see: J. Am. Chem. Soc. 2017, 139, 4062, and highlight in Chem. & Eng. News, 2017, 95, 11. Our nitrogen Lewis acids were selected as molecules of the year (2017) by CEN (Chem. & Eng. News, 2017, 95, 49).
First example of Nitrogen-based Lewis acid. Lewis acids and their interactions are among the most fundamental facets of chemistry. Many chemical elements can serve as the central atom of Lewis acids (e.g., boron, aluminum, phosphorous, etc.). Nitrogen is associated with Lewis bases due to a lone pair of electrons. We discovered a first X-ray supported example of Lewis acids centered on nitrogen atom. Our novel class of Lewis acids is robust, stable and stereoelectronically modifiable, which make them as a very attractive platform for their employment in Lewis acid catalysis, frustrated Lewis pairs chemistry, small molecules activation, etc. Based on the reactivity of these new Lewis acids, we prepared, for the first time, cyclic triazanes – a class of organic compounds sequentially bearing three all-saturated nitrogen atoms (N-N-N motif). We envision very interesting and unusual chemistry (which is under intensive investigation in our labs) of this novel class of nitrogen materials. For more details, see: J. Am. Chem. Soc. 2017, 139, 4062, and highlight in Chem. & Eng. News, 2017, 95, 11. Our nitrogen Lewis acids were selected as molecules of the year (2017) by CEN (Chem. & Eng. News, 2017, 95, 49).
-
- Decarboxylative Halogenations and Asymmetric Catalytic Synthesis of Organic Fluorides
This is a second major direction in the group. We design and develop new efficient methods and reagents for synthesis of organic halides and their utilization in the new catalytic transformations for the preparation of complex organic structures (focused on organic fluorides) with potential interest in pharmaceutical and agrichemical areas.
-
-
 Efficient and practical iodo-, bromo- and chloro-decarboxylation processes. Organic halides represent a major class of chemical materials with indispensable use as final products and intermediates in synthesis. Therefore, methods for their selective and efficient preparation are of great importance. Direct and selective conversion of widely available and inexpensive carboxylic acids (chemical feedstock) to corresponding valuable organic halides, so-called halo-decrboxylation, is a highly attractive approach. We have developed a highly attractive technology for the direct production of valuable organic halides from widely available and inexpensive carboxylic acids. Three separate methods – iodo-, bromo- and chloro-decarboxylation – were developed in our labs. We thoroughly studied the mechanisms of these processes and demonstrated a beneficial application of our methods by preparing hundreds of diverse organic halides (including aliphatic (primary, secondary and tertiary) and aromatic halides). Our one-pot method is efficient, robust and economical: no use of heavy poisonous metals or multistep procedures with tedious separations are needed. We truly believe that our halo-decarboxylations are among the most general and practical methods for such type of transformations and, hopefully, will be a method-of-choice in synthetic community. These studies were partially published in Adv. Syn. & Cat., 2011, 353, 1438, J. Org. Chem. 2017, 82, 7093 (see also highlight in Chem. & Eng. News, 2017, 95, 9); US Patent 9,637,462; US Patent App. 15/763,848; US Patent App. 15/763,846.
Efficient and practical iodo-, bromo- and chloro-decarboxylation processes. Organic halides represent a major class of chemical materials with indispensable use as final products and intermediates in synthesis. Therefore, methods for their selective and efficient preparation are of great importance. Direct and selective conversion of widely available and inexpensive carboxylic acids (chemical feedstock) to corresponding valuable organic halides, so-called halo-decrboxylation, is a highly attractive approach. We have developed a highly attractive technology for the direct production of valuable organic halides from widely available and inexpensive carboxylic acids. Three separate methods – iodo-, bromo- and chloro-decarboxylation – were developed in our labs. We thoroughly studied the mechanisms of these processes and demonstrated a beneficial application of our methods by preparing hundreds of diverse organic halides (including aliphatic (primary, secondary and tertiary) and aromatic halides). Our one-pot method is efficient, robust and economical: no use of heavy poisonous metals or multistep procedures with tedious separations are needed. We truly believe that our halo-decarboxylations are among the most general and practical methods for such type of transformations and, hopefully, will be a method-of-choice in synthetic community. These studies were partially published in Adv. Syn. & Cat., 2011, 353, 1438, J. Org. Chem. 2017, 82, 7093 (see also highlight in Chem. & Eng. News, 2017, 95, 9); US Patent 9,637,462; US Patent App. 15/763,848; US Patent App. 15/763,846. -
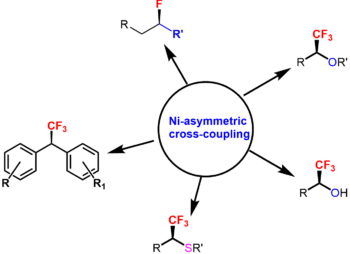 Asymmetric Catalytic Methods for Synthesis of Organic Fluorides. Methods for selective introduction of fluorine or fluorinated groups in organic molecule, especially in its eneatioselective manner, are of high demand for discovery and preparation of new drugs, agrichemicals and liquid crystals. Our novel halo-decarboxylation processes allowed for a preparation of libraries of geminal halo(fluoro)-alkanes. These unique polyfunctional compounds were previously underestimated in synthesis, likely, due to a lack of practical methods for their preparation. We employed these and related substrates for the development of new catalytic asymmetric syntheses of highly valuable organic fluorides. We developed methods for the preparation of chiral fluoro-organic materials, including secondary fluoroalkanes, CF3-substituted alcohols, ethers, thioethers and benzhydryls. The developed technologies are based on inexpensive Ni catalysis, and allow for a rapid site-selective fluorination of organic materials. During our studies, we discovered, for the first time, an employment of organo-titanium compounds as nucleophiles in asymmetric cross-couplings. We currently focus our active program in this direction to mechanistic understanding of these processes and further development of these discoveries. For more details, see: J. Am. Chem. Soc. 2014, 136, 1305; J. Am. Chem. Soc. 2015, 137, 2542; Chem. Sci., 2016, 7, 2762; Nature Commun. 2018, 9, 3566; J. Am. Chem. Soc. 2019, 141, 10994.
Asymmetric Catalytic Methods for Synthesis of Organic Fluorides. Methods for selective introduction of fluorine or fluorinated groups in organic molecule, especially in its eneatioselective manner, are of high demand for discovery and preparation of new drugs, agrichemicals and liquid crystals. Our novel halo-decarboxylation processes allowed for a preparation of libraries of geminal halo(fluoro)-alkanes. These unique polyfunctional compounds were previously underestimated in synthesis, likely, due to a lack of practical methods for their preparation. We employed these and related substrates for the development of new catalytic asymmetric syntheses of highly valuable organic fluorides. We developed methods for the preparation of chiral fluoro-organic materials, including secondary fluoroalkanes, CF3-substituted alcohols, ethers, thioethers and benzhydryls. The developed technologies are based on inexpensive Ni catalysis, and allow for a rapid site-selective fluorination of organic materials. During our studies, we discovered, for the first time, an employment of organo-titanium compounds as nucleophiles in asymmetric cross-couplings. We currently focus our active program in this direction to mechanistic understanding of these processes and further development of these discoveries. For more details, see: J. Am. Chem. Soc. 2014, 136, 1305; J. Am. Chem. Soc. 2015, 137, 2542; Chem. Sci., 2016, 7, 2762; Nature Commun. 2018, 9, 3566; J. Am. Chem. Soc. 2019, 141, 10994.
-